DIR Technologies Partners with Pfizer
May 13, 2015
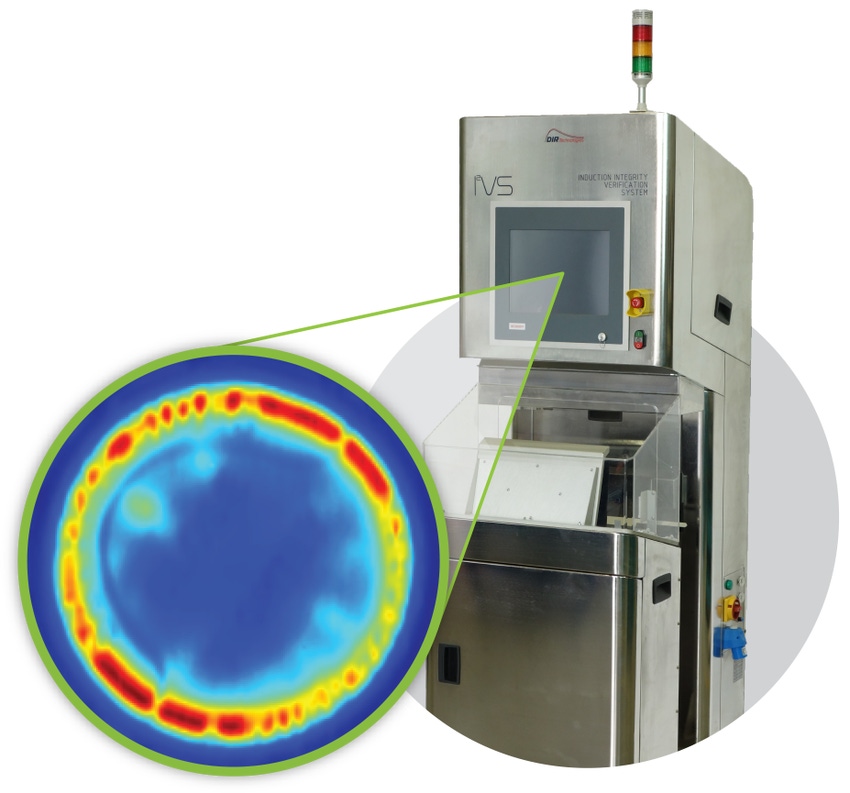
DIR Technologies, a provider of quality and process control solutions for pharmaceutical primary packaging, has announced a five-year agreement with Pfizer Inc.
The agreement provides Pfizer with access to DIR Technologies’ first-to-industry inspection and quality control solutions, and sets the stage for joint projects pertaining to both product production and packaging line QA/QC processes.
Among the solutions involved in the agreement is DIR Technologies’ flagship Induction Integrity Verification System (I2VS). Introduced just over a year ago, the system is the first in the global pharmaceuticals market to perform in-line inspection on 100% of induction sealed bottles at the speed of the machine throughput. The I2VS is a workflow solution as well as an inspection device: the source of any potential faulty seals is indicated in real time, yielding a high degree of process understanding and the ability for operators to not only help fix potential faulty seals immediately, but oftentimes prevent them from occurring in the first place.
“Obviously, Pfizer is a well-established leader in pharmaceuticals manufacturing and part of our excitement in working with Pfizer is the company’s dedication to also being an industry pioneer in terms of technological advancement,” said Roni Mansur, CEO of DIR Technologies. “Pfizer’s methodology of continuous improvement drives it to test and implement the best of cutting-edge solutions. It takes a true entrepreneurial spirit to lead the way and make a change, to implement technology that is different from the industry norm.”
Whereas sampling has been the standard methodology, the I2VS enables a new level of quality assurance and process control. After an extensive trial, Pfizer recently decided to adopt DIR’s inspection technology on select packaging lines.
DIR Technologies and Pfizer also will be working together on the development of a new inspection technology based on DIR’s advanced high-speed imaging capabilities. The new system is intended to perform 100 percent in-line inspection of tablets and capsules.
For related articles, news, and equipment reviews, visit our Equipment Zones
Click here for information about the upcoming Powder & Bulk Solids Canada conference/exhibition
You May Also Like


